The UK government has unveiled an ambitious plan to phase out animal testing for drug development, marking a significant shift in how medicines are developed and tested. This groundbreaking initiative aims to enhance animal welfare while simultaneously promoting innovation in the life sciences sector, potentially transforming how veterinary medicines and treatments for pets are developed in the future.
The UK animal testing phase-out represents one of the most comprehensive efforts globally to reduce reliance on animal models in pharmaceutical research. For pet owners, this development signals a new era where the medicines that keep their beloved companions healthy may be developed through more humane and potentially more effective methods.
Understanding the UK's Animal Testing Phase-Out Initiative
The government's announcement outlines a strategic approach to gradually reduce and eventually eliminate the need for animal testing in drug development. This initiative recognizes both the ethical concerns surrounding animal testing and the scientific advances that now offer viable alternatives to traditional animal-based research methods.
Animal welfare in science has become an increasingly important consideration as public awareness grows about the conditions and procedures involved in traditional testing. The UK's plan acknowledges these concerns while ensuring that the safety and efficacy of medicines—including those for pets—remain uncompromised.
Revolutionary Animal Testing Alternatives Leading the Way
The phase-out plan heavily emphasizes the development and implementation of non-animal testing methods that have shown remarkable promise in recent years. These alternatives to animal testing include several cutting-edge technologies that may actually provide more accurate results than traditional animal models.
Organ-on-a-Chip Technology
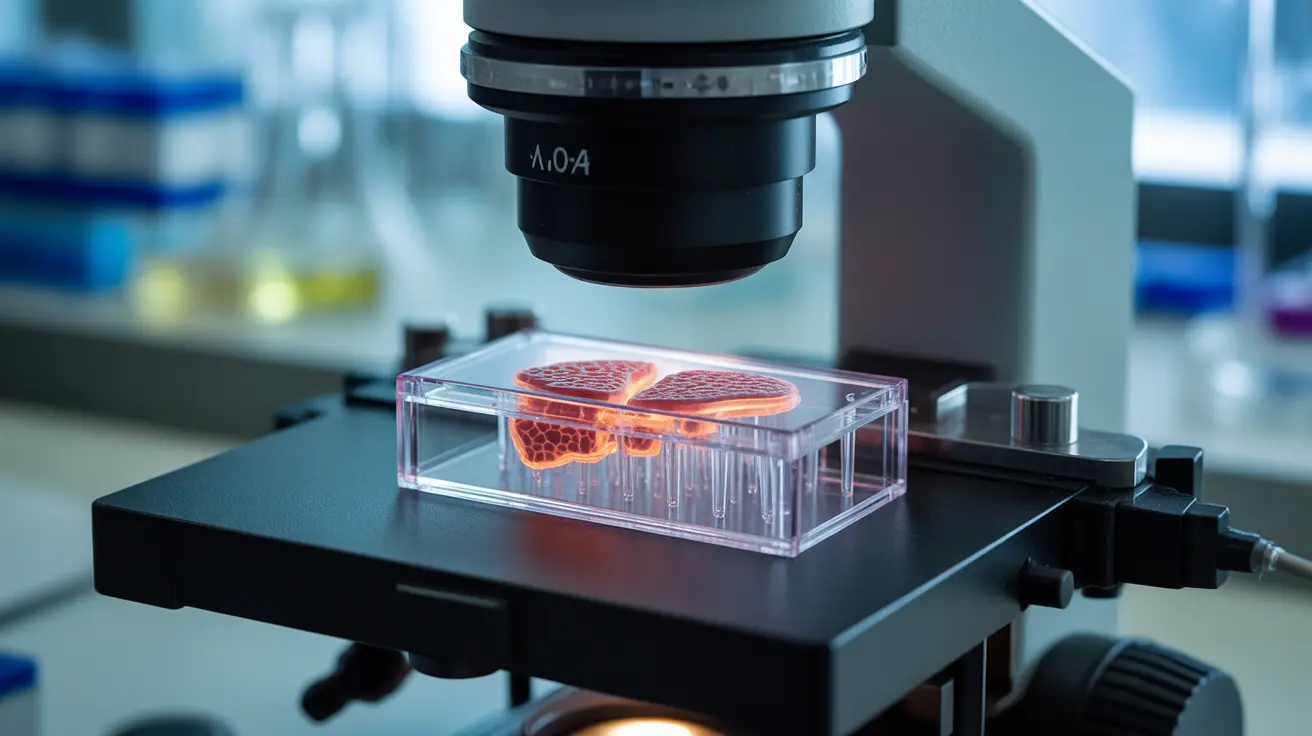
One of the most promising alternatives to animal testing for pets and humans alike is organ-on-a-chip technology. These sophisticated devices recreate the functions of human and animal organs on microchips, allowing researchers to observe how drugs interact with living tissue without using live animals. This technology offers unprecedented precision in predicting how treatments will affect pets' organs and systems.
3D Bioprinted Tissues and AI Integration
The plan also highlights the role of 3D bioprinted tissues in creating realistic models for drug testing. When combined with AI in drug testing, these technologies can process vast amounts of data to predict drug interactions and effects with remarkable accuracy. These methods often provide more relevant results for veterinary medicine safety than traditional animal testing.
Timeline and Implementation of Animal Testing Regulations UK
While the government has announced its commitment to this transformative approach, the animal testing timeline UK follows a measured approach that ensures safety standards are maintained throughout the transition. The phase-out will be gradual, allowing time for alternative methods to be fully validated and regulatory frameworks to be updated.
The establishment of the UKCVAM centre (UK Centre for Validation of Alternative Methods) will play a crucial role in replacing animal testing. This center will validate new testing methods and ensure they meet the rigorous standards required for drug approval, including veterinary medicines.
Impact on Veterinary Medicine and Pet Healthcare
For pet owners, this shift toward animal-free drug testing could lead to more targeted and effective treatments for their companions. Alternative testing methods often provide more species-specific data, potentially resulting in better outcomes for pets receiving these medications.
The transition also means that future veterinary medicines may be developed more quickly and cost-effectively, as alternative methods can often produce results faster than traditional animal studies. This efficiency could translate to more affordable treatments for pet owners.
Supporting Innovation While Ensuring Safety
The UK animal testing ban approach emphasizes that innovation and safety must go hand in hand. The government recognizes that maintaining public and professional confidence in pharmaceutical products requires rigorous testing standards, whether using animals or alternative methods.
Pet owners can take comfort in knowing that this transition prioritizes both animal welfare and the continued development of safe, effective treatments for their beloved companions.
Frequently Asked Questions
What does the UK's animal testing phase-out plan mean for pet owners?
The phase-out plan means that future veterinary medicines may be developed using more humane and potentially more accurate testing methods. Pet owners can expect continued access to safe, effective treatments while supporting better animal welfare practices in research.
How will ending animal testing affect the safety of medicines for pets?
Safety standards will remain just as rigorous through alternative testing methods like organ-on-a-chip technology and AI-driven analysis. These methods often provide more precise and relevant data than traditional animal testing, potentially leading to safer medications for pets.
When will animal testing for pet medicines be reduced or stopped in the UK?
The timeline follows a gradual approach, allowing alternative methods to be fully validated and integrated into regulatory frameworks. The transition will be carefully managed to ensure no compromise in safety or effectiveness of veterinary medicines.
Looking Toward a More Humane Future
The UK's commitment to phasing out animal testing represents a significant step forward in balancing scientific progress with ethical considerations. For the pet care community, this initiative promises a future where the medicines that keep our companions healthy are developed through methods that align with our values of compassion and care for all animals.
As these alternative methods continue to evolve and prove their effectiveness, pet owners can feel confident that their companions will benefit from both innovative treatments and the knowledge that these advances were achieved through humane research practices.