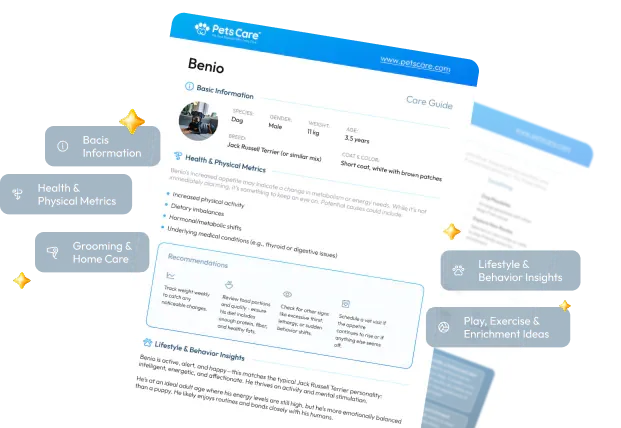
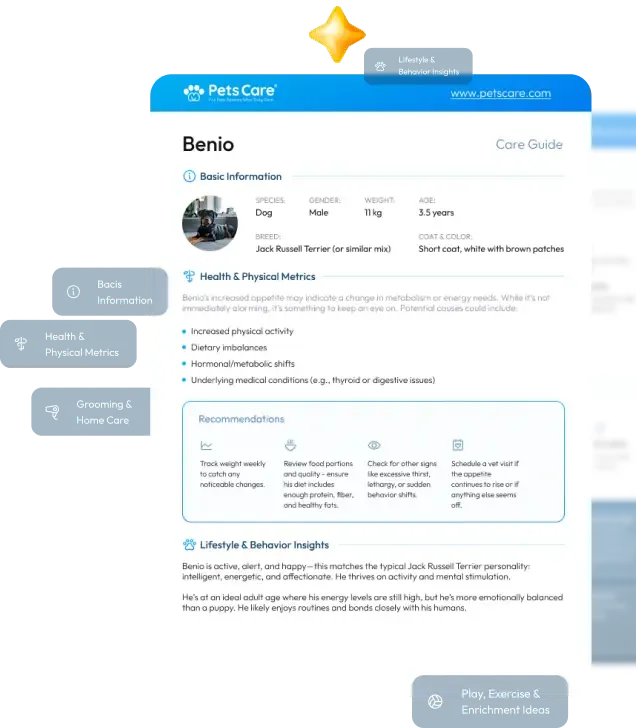
Can Fenbendazole Be Used in Humans? Understanding Its Potential and Risks
Fenbendazole is a well-established veterinary medication known primarily for its ability to combat intestinal parasites in animals such as dogs, cats, livestock, and some exotic species. But in recent years, there’s been growing curiosity—particularly amidst anecdotal reports and internet forums—about its potential application in humans, especially in relation to cancer treatment. So, can fenbendazole be safely used in humans?
What is Fenbendazole?
Fenbendazole is an anthelmintic agent belonging to the benzimidazole class of drugs. It works by disrupting microtubule-dependent functions in parasitic organisms, effectively starving and eliminating them. In veterinary practice, it treats:
- Roundworms
- Hookworms
- Whipworms
- Some tapeworms (e.g., Taenia spp.)
- Protozoa like Giardia
It is highly effective due to its localized action in the intestinal tract, amplified when given with food to increase absorption.
FDA Approval and Human Usage
While **fenbendazole** has shown promise in animal care, it is **not approved by the FDA for human use**. Other benzimidazoles—like **mebendazole and albendazole**—are commonly prescribed for human parasitic infections. Fenbendazole remains in use primarily within veterinary medicine due to a lack of comprehensive human clinical trials.
Exploration in Cancer Research
Scientific curiosity about fenbendazole stems from **preclinical studies** showing it may have **antiproliferative effects on various cancer cell lines**. Proposed mechanisms include:
- Microtubule destabilization—interfering with cell division
- Inhibition of GLUT1 and hexokinase—reducing glucose uptake and glycolysis
- Induction of apoptosis—promoting programmed cell death
- Oxidative stress modulation
However, despite these promising laboratory findings, no clinical trials have definitively established its efficacy or safety in human populations.
Self-Medication and Safety Concerns
Anecdotal reports often highlight patients with cancer who self-medicate with fenbendazole in hopes of benefiting from its speculative anticancer properties. While some claim tumor regression and improved quality of life, there are also documented risks:
- Hepatotoxicity—liver inflammation has been reported
- Variable dosing—veterinary formulations are not standardized for humans
- Limited absorption—bioavailability remains a concern in oral human administration
Without medical supervision, these risks increase, especially as the drug interacts with other medications or health conditions.
Pharmacokinetics and Bioavailability
In humans, **fenbendazole's poor absorption and quick metabolism** limit its systemic reach, making it less effective for conditions requiring systemic treatment. Studies on **oxfendazole**, one of its metabolites, in human volunteers suggest a more favorable profile, but this too remains under study and is not fully approved for general medical use.
Veterinary Safety Profile
In animals, fenbendazole has been shown to be:
- Safe and well-tolerated when administered as directed
- Rarely associated with severe side effects
- Effective at recommended doses for several parasite species
This established safety in veterinary contexts does not automatically translate into safe use in humans, where metabolic pathways and tolerance thresholds may greatly differ.
Current Medical Consensus
Experts and regulatory bodies generally advise against the off-label use of fenbendazole in humans due to:
- Lack of human clinical trial data
- Insufficient regulatory oversight
- Potential safety risks and side effects
Its use remains confined to experimental settings and should never replace approved medical treatments for conditions such as cancer or parasitic diseases.
Conclusion
While the science behind fenbendazole’s mechanism opens the door to potential human applications, current evidence is far from sufficient to warrant its safe consumption by people. **It is not FDA-approved for human use**, and any attempts to self-medicate with veterinary drugs may lead to harm. People interested in novel therapeutic options should consult with a healthcare provider and rely on evidence-backed, clinically approved treatments.